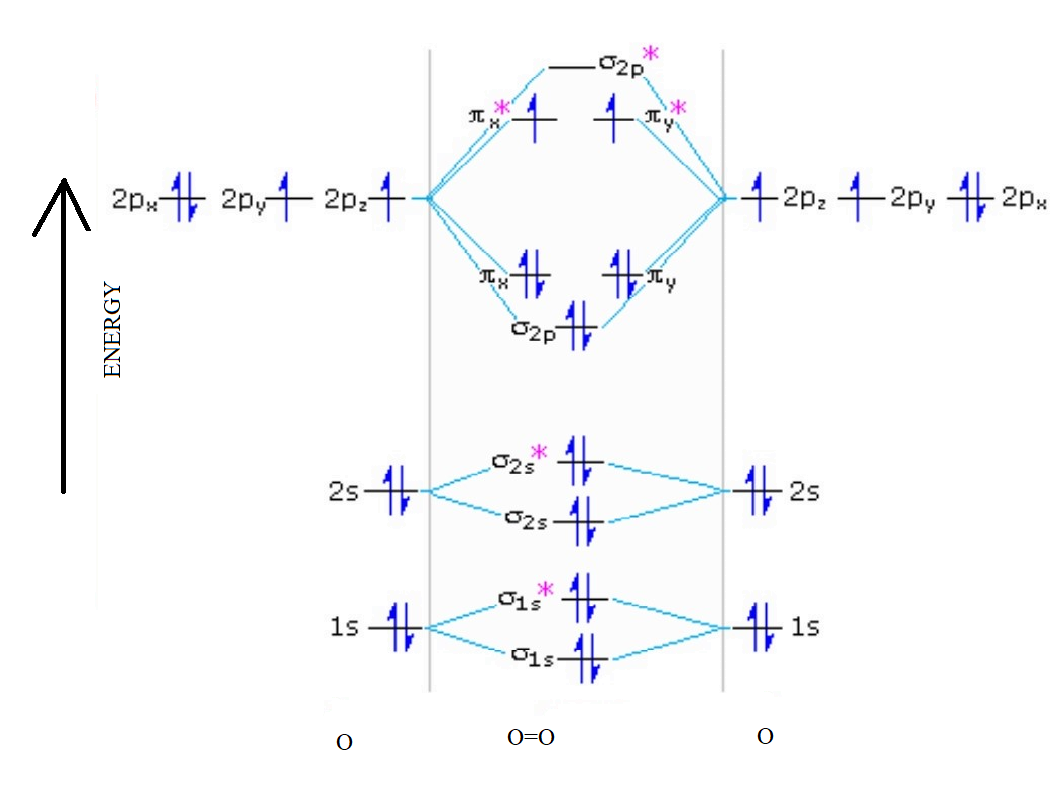
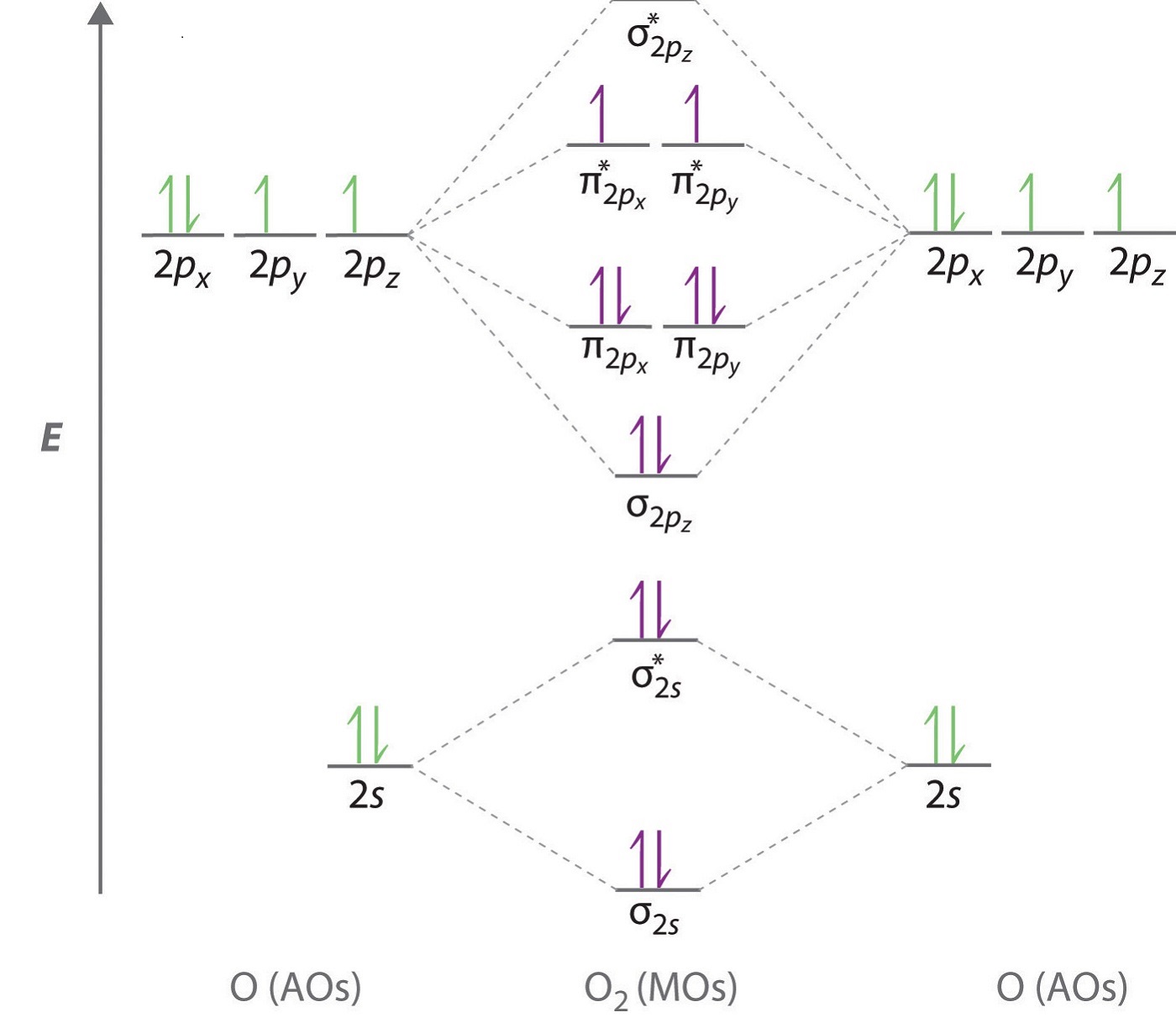
Solution. The bond length in the oxygen species can be explained by the positions of the electrons in molecular orbital theory. To obtain the molecular orbital energy-level diagram for \(\ce{O2}\), we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in Figure 9.10.1 . To write the orbital diagram for the Oxygen atom (O) first we need to write the electron configuration for just O. To do that we need to find the number of electrons for the Oxygen atom.
Oxygen Electron Configuration (O) with Orbital Diagram
Oxygen has one more electron than Nitrogen and as the orbitals are all half filled the electron must pair up.. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. This is done by first determining the subshell (s,p,d, or. As of December 2014, up to 46% of the energy in sunlight could be converted into electricity using solar cells. Example 6.9.2: M olecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons. Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. From this diagram, calculate the bond order for [latex]\ce{O2}[/latex]. How does this diagram account for the paramagnetism of [latex]\ce{O2}[/latex]? Show Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 7.7.12. Each oxygen atom contributes six electrons, so the diagram appears as shown in Figure 7.7.15. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s < p < d < f. Figure 6.24 depicts how these two trends in increasing energy relate.
Explain the formation of {O_2} molecule using molecular orbital theory.
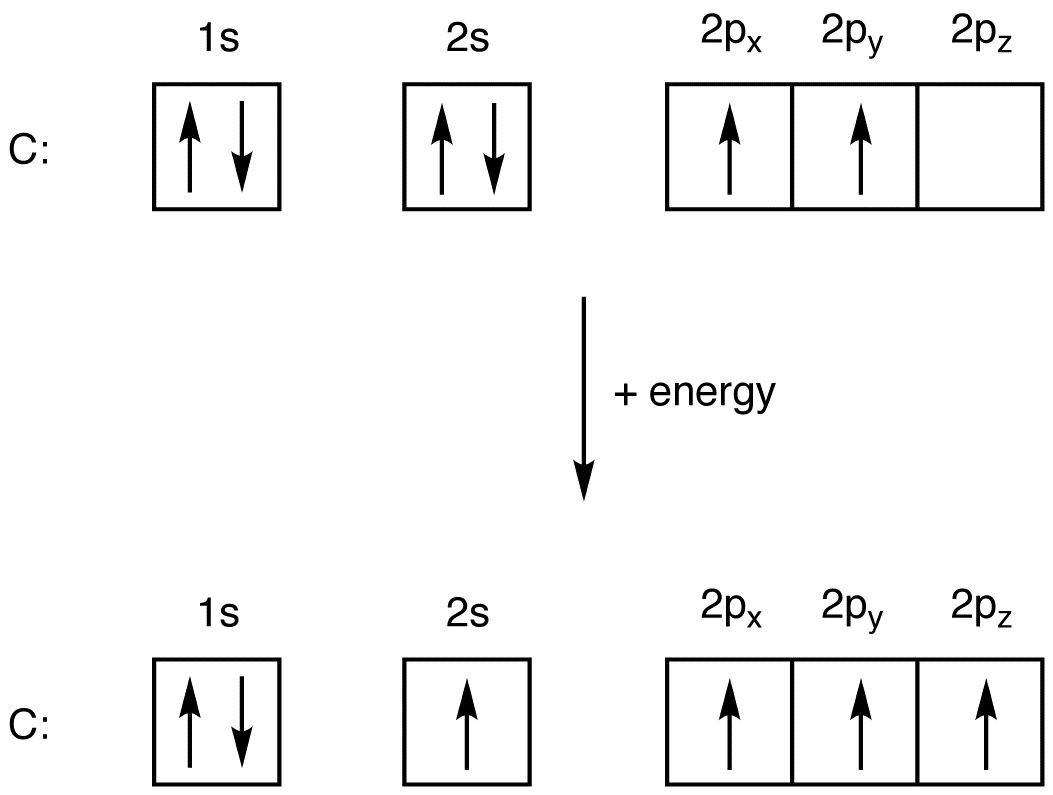
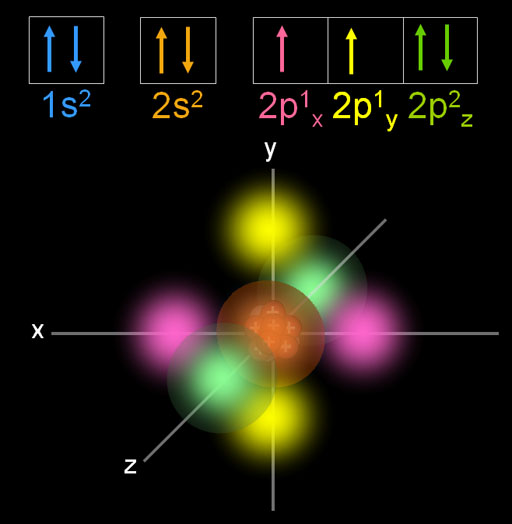
Oxygen is the eighth element with a total of 8 electrons. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be. Oxygen orbital diagram October 20, 2023 by Deep The information on this page is fact-checked. Oxygen orbital diagram The orbital diagram of oxygen shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, and the 2p subshell has 4 electrons. Contents Steps Find electrons Write electron configuration Draw orbital diagram Related A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 8.3. 3 or 8.3. 4 ). Thus, the electron configuration and orbital diagram of lithium are:
O2 Molecular Orbital Diagrams 101 Diagrams
Oxygen is the 8th element in the periodic table and its symbol is 'O'. In this article, I have discussed in detail how to easily write the complete electron configuration of oxygen. What is the electron configuration for oxygen? The total number of electrons in oxygen is eight. When two oxygen atoms overlap, the sigma (2p) molecular orbital is LOWER in energy than the pi (2p) orbitals. This different from Nitrogen, where it's the othe.
The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps! In O 2, therefore, we need to accommodate twelve valence electrons (six from each oxygen atom) in molecular orbitals. As you can see from the diagram, this places two electrons in antibonding orbitals. Each of these electrons occupies a separate π* orbital because this leads to less electron-electron repulsion (Hund's Rule).
Electron Configuration
The left-hand side diagram is of O2 at ground level whereas the right-hand side diagram is of rearranged electrons as per the Lewis structure within the O2 molecule. It takes a lot of energy to pair up the electrons within the same orbital. So, the diagram having no unpaired electrons is at higher energy. It means it is at a much higher excited. Electronic structure Singlet oxygen refers to one of two singlet electronic excited states. The two singlet states are denoted 1 Σ + g and 1 Δ g (the preceding superscript "1" indicates a singlet state). The singlet states of oxygen are 158 and 95 kilojoules per mole higher in energy than the triplet ground state of oxygen.




