Learn to determine if SO42- is polar or nonpolar based on the polarity between bonds and the molecular geometry (shape).Ions, like SO42- (sulfate) are someti. Is SO42- Polar or Nonpolar? (Sulfate Ion) Geometry of Molecules 2.71K subscribers 5 679 views 1 year ago Polarity of Molecules Hello Guys! SO42- ion or Sulphate ion's polarity is quite.
SO4 2 Molecular Geometry / Shape and Bond Angles YouTube
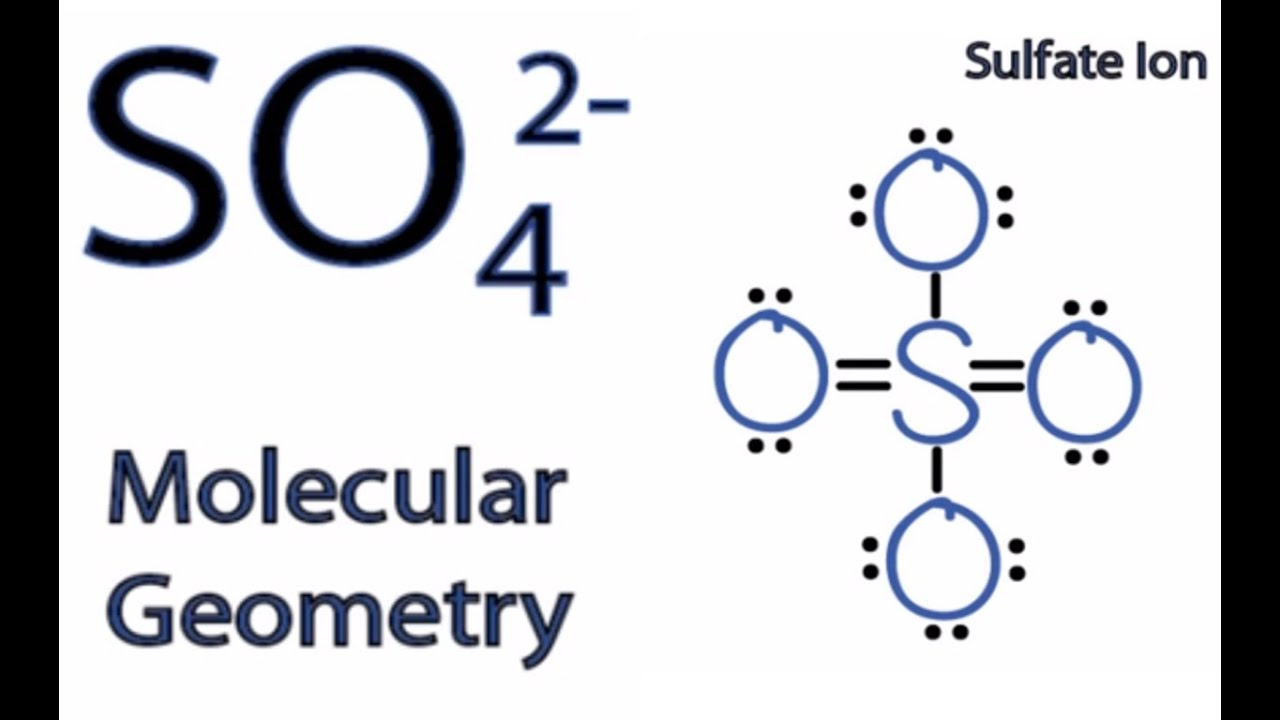
SO42- is a chemical formula for Sulfate ion; it comprises one Sulfur Atom and four oxygen atoms. It also has a -2 charge because of the additional electrons it accepts to attain this structure. This blog post will help you understand if this ion is polar or nonpolar, although a -2 charge might confuse you. Sulfate ion (SO42-) is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of -2. We find sulfates in a wide range of compounds, some of the well-known being MgSO4, CaSO4, Na2SO4, and PbSO4. We can easily prepare sulfates via oxidizing metal sulfites and sulfides. SO42- is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of -2. It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates, and much more. It is also a sulfate salt for sulphuric acids. SOX4X2− S O X 4 X 2 − is a polyatomic ion as well as a nonpolar covalent compound. How can it be ionic and covalent at the same time? Same applies to sulfite SOX3X2− S O X 3 X 2 −. It's a polyatomic ion as well as a polar covalent bond. How? bond ions covalent-compounds polarity Share Cite Improve this question Follow edited Apr 22, 2021 at 21:20

Lewis Structure SO4 2 plus dipoles, shape, angles, resonance and
Sulfate is a very weak oxidizing agent. Since sulfur is in its maximum oxidation number in sulfate ion, this ion cannot act as a reducing agent. This page titled Sulfate Ion (SO₄²⁻) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by James P. Birk. Sulfate ion is a very weak base. Draw the most important Lewis structure for [ SO4 ]2− and then answer the following questions. The underlined atom is the central atom. All other atoms are bonded directly to the central atom. (a) What is the electron-group geometry, according to VSEPR theory? (b) What is the molecular geometry? (c) Is this species polar or nonpolar? Notice that a tetrahedral molecule such as CCl4 CCl 4 is nonpolar Figure ( 4.14.1 4.14. 1. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Figure 4.14.1 4.14. 1 Some examples of nonpolar molecules based on molecular geometry (BF 3 and CCl 4 ). Henry Agnew (UC Davis) 5.10: Electronegativity and Bond Polarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken if energy is added to a molecule.

Is SF4 Polar or Nonpolar? (Sulfur Tetrafluoride) YouTube
Figure \(\PageIndex{6}\): The molecular geometry of a molecule affects its polarity. CO 2 is a nonpolar molecule, while H 2 O is a polar molecule. It should be noted that if the all of the bonds in a molecule are nonpolar, then the molecule will be nonpolar. Consider a molecule of O 2, . The double bond between the two oxygen atoms is a. SO4^2-: - The 3D sketch for SO4^2- is a tetrahedral shape, with bond angles of approximately 109.5 degrees. AsF5: - The 3D sketch for AsF5 is a trigonal bipyramidal shape, with bond angles of approximately 90 and 120 degrees. Answer Step 3: Finally, we need to determine if each molecule is polar or nonpolar.
Each C-O bond in CO 2 is polar, yet experiments show that the CO 2 molecule has no dipole moment. Because the two C-O bond dipoles in CO 2 are equal in magnitude and oriented at 180° to each other, they cancel. As a result, the CO 2 molecule has no net dipole moment even though it has a substantial separation of charge. Page Contents show How to draw lewis structure of SO42-? The Lewis structure of a sulfate [SO4]2- ion consists of 1 sulfur (S) atom and 4 atoms of oxygen (O). The sulfur atom is present at the center of the Lewis structure while the oxygen atoms occupy terminal positions.

Lewis Dot Diagram For So4 2
Answer = SO4 2- ( sulfate ) is Nonpolar What is polar and non-polar? Polar "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Learn to determine if SO2 (Sulfur dioxide) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Stru.




