239 32K views 9 years ago This video shows how to draw the orbital diagram of Sodium (Na). It also shows how to write the electron configuration of Sodium (Na) and the shorthand noble gas. The sodium orbital diagram is a graphical representation of the electron configuration of the sodium atom. This diagram shows how the electrons in the sodium atom are arranged in different orbitals. Orbital is the region of space around the nucleus of an atom where electrons are found.
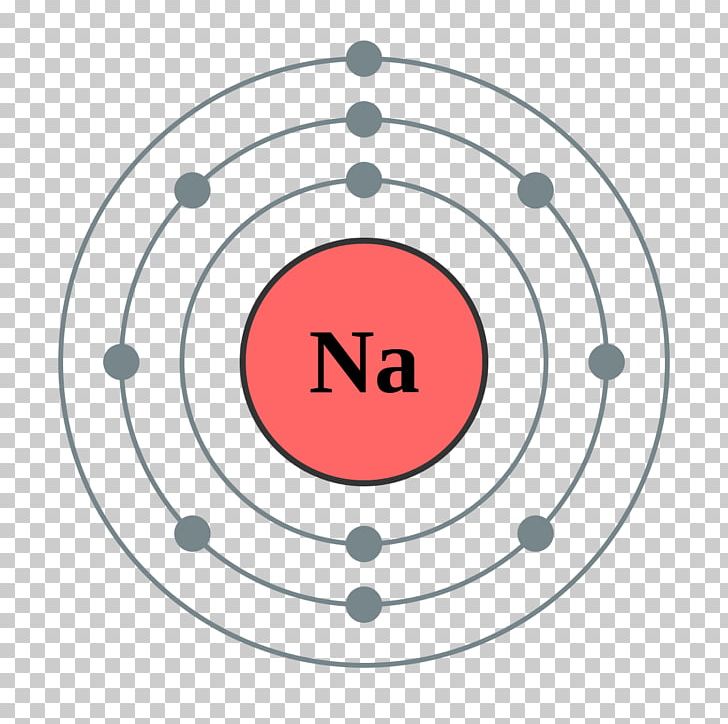
Electron Shell Sodium Electron Configuration Bohr Model PNG, Clipart
To write the orbital diagram for the Sodium atom (Na) first we need to write the electron configuration for just Na. To do that we need to find the number o. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (Table \(\PageIndex{1}\)). The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon \(\left( Z=10 \right)\).. An orbital diagram is the more visual way. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s < p < d < f. Figure 6.24 depicts how these two trends in increasing energy relate. Referring to either Figure 2.6.3 2.6. 3 or 2.6.4 2.6. 4, we would expect to find the electron in the 1 s orbital. By convention, the ms = +1 2 m s = + 1 2 value is usually filled first. The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2.
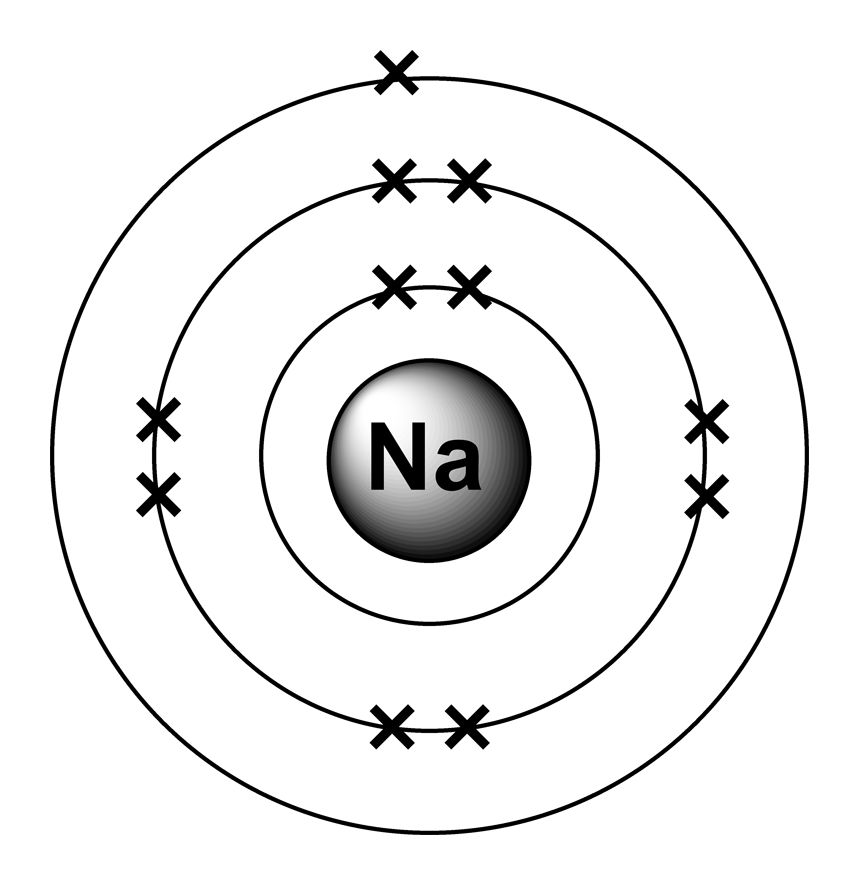
Sodium Table of Elements by Shrenil Sharma
In order to write the Na electron configuration we first need to know the number of electrons for the Na atom (there are 11 electrons). When we write the configuration we'll put all 11 electrons in orbitals around the nucleus of the Sodium atom. In writing the electron configuration for sodium the first two electrons will go in the 1s orbital. Orbital Diagrams. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. Finally, the third shell would have a smaller circle with one arrow pointing up, representing the single electron in the 3s orbital. Sodium Electron Configuration Diagram. Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white metal that is highly reactive and can easily form compounds with other elements. The Na 3s 3 s orbital combines with Cl 3pz 3 p z to form the molecular orbitals labeled 4σ 4 σ and 4σ∗ 4 σ ∗ in Figure 5.3.3.1 5.3.3. 1. The 4σ 4 σ orbital is weakly bonding, but is very close in energy to the Cl 3pz 3 p z orbital, and is mostly Cl-like in character. Notice that all σ σ orbitals look very much like either s s or p p.
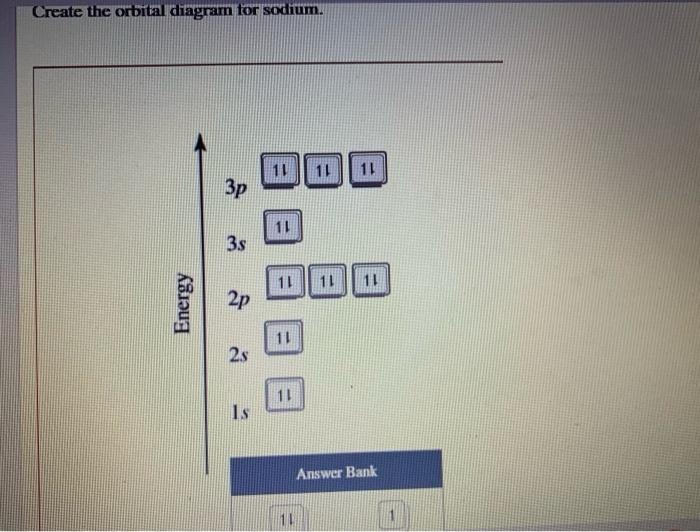
Solved Create the orbital diagram for sodium. 11 11 11 Зр 11
In this video we will write the electron configuration for Na+, the Sodium ion. We'll also look at why Sodium forms a 1+ ion and how the electron configurati. 0:00 / 2:00 Orbital Diagrams: Sodium Conrad Capule 69 subscribers Subscribe 12 1.8K views 2 years ago In this video, we determine how to draw the orbital diagram of sodium..more.more.
A Sodium atom is a neutral atom that has an atomic number of 11 which implies it has a total of 11 electrons. As per the Aufbau rule, the electrons will be filled into 1s orbital first then 2s, then 2p…so on. Sodium Electron Configuration (Na) with Orbital Diagram. Sodium Electron Configuration: The chemical element sodium has the symbol Na and atomic number 11. This is soft, reactive, silver + whitish metal. Electron configuration can define as the distribution of electrons of molecules or atoms in molecular or atomic orbits.
Aufbau Diagram For Sodium
C We obtain the valence electron configuration by ignoring the inner orbitals, which for phosphorus means that we ignore the [Ne] closed shell. This gives a valence-electron configuration of 3 s2 3 p3. Exercise 6.8.1 6.8. 1. Draw an orbital diagram and use it to derive the electron configuration of chlorine, Z = 17. Sodium is the 11th element in the periodic table and its symbol is 'Na'. Sodium is a classified alkali metal element. In this article, I have discussed in detail how to easily write the complete electron configuration of sodium. What is the electron configuration for sodium? The total number of electrons in sodium is eleven.




