Lewis Structure of CS2 If you are a student of chemistry, it is almost obvious that you are aware of the term 'Lewis Structure". If not, here's a brief explanation of the above-mentioned topic. Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure. A step-by-step explanation of how to draw the CS2 Lewis Dot Structure (Carbon disulfide).For the CS2 structure use the periodic table to find the total numbe.

CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
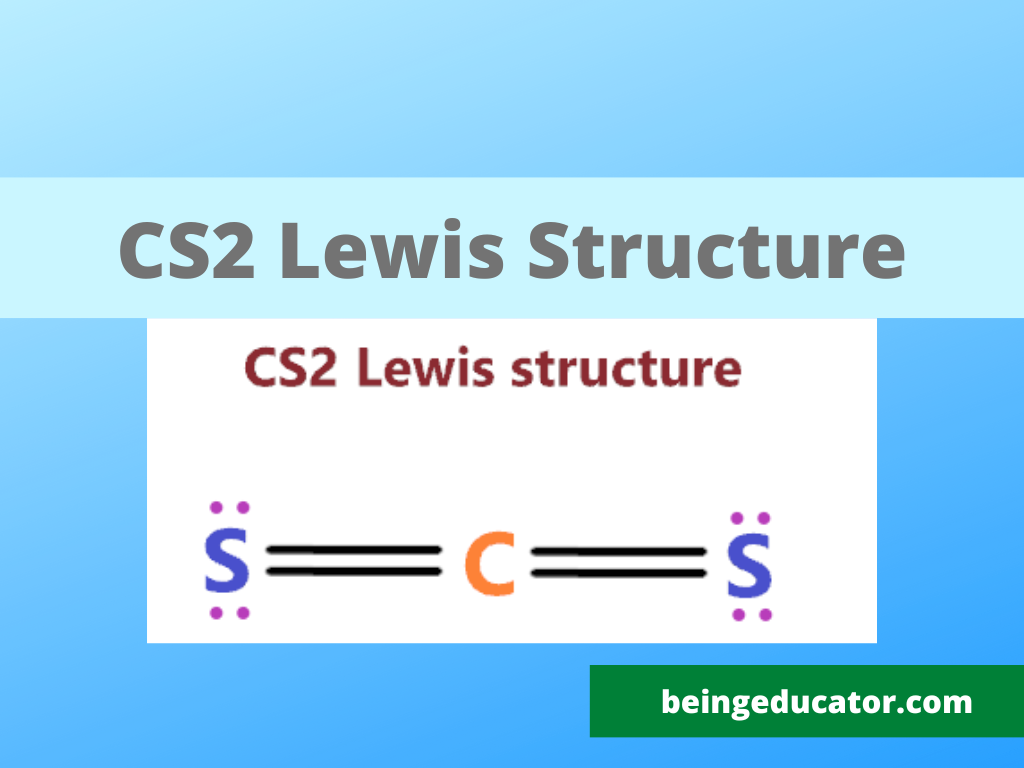
In Lewis structure of CS2 molecule, there are 16 valence electrons, out of which four valence electrons are of Carbon, and six valence electrons are from each sulfur molecule. Carbon is the least electronegative molecule and thus comes in the center. These two sulfur molecules form double bonds with this Carbon molecule to complete Carbon's octet. CS2 Lewis Structure The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na - 1s 2 2s 2 2p 6 3s 1, Cl - 1s 2 2s 2 2p 6 3s 2 3p 5 A step-by-step explanation of how to draw the CS2 Lewis Dot Structure (Carbon disulfide).For the CS2 structure use the periodic table to find the total numbe. 6 steps of CS2 Lewis structure: Step 1: Total number of valence electrons: Step 2: Determine the central metal atom: Step 3: Connect the atoms with dots: Step 4: Distribution of remaining valence electrons: Step 5: Check if all atoms have an octet: Step 6: Formal charge: Synthesis/Production: Direct Synthesis:
CS2 Lewis Structure Molecular Geometry Polarity Hybridization
In the CS 2 Lewis structure, there are two double bonds around the carbon atom, with two sulfur atoms attached to it, and on each sulfur atom, there are two lone pairs. CS2 Lewis Structure: How to Draw the Lewis Structure for CS2 Watch on Contents Steps #1 First draw a rough sketch #2 Mark lone pairs on the atoms Lewis structure of CS2 (or Carbon Disulfide) contains two double bonds between the Carbon (C) atom and each Sulfur (S) atom. The Carbon atom (C) is at the center and it is surrounded by 2 Sulfur atoms (S). The Carbon atom does not have a lone pair while both the Sulfur atoms have 2 lone pairs. A quick explanation of the molecular geometry of CS2 including a description of the CS2 bond angles.Looking at the CS2 Lewis structure we can see that there. For the CS2 Lewis structure, calculate the total number of valence electrons for the CS2 molecule. After determining how many valence electrons there are in CS2, place them around the central atom to complete the octets. There are 16 valence electrons for the CS2 Lewis structure.
CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Drawing the Lewis Structure for CS 2 ( Sulfur Trioxide) CS 2 is sometimes used to fumigate railroad cars and grain elevators. CS 2 is named Carbon Disulfide. There are 16 valence electrons available for the Lewis structure for CS 2 . Try to draw the CS 2 Lewis structure before watching the video. Added Jun 9, 2014 by WebTester in Chemistry This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
To draw the Lewis structure for CS2, follow these steps: 1. Calculate the total number of valence electrons in CS2: Carbon (C) possesses 4 valence electrons, while sulfur (S) has 6 valence electrons. As there are two sulfur atoms, the total valence electrons in CS2 sum up to 16. 2. Identify the central atom, which is the least electronegative atom. Steps of drawing CS2 lewis structure Step 1: Find the total valence electrons in CS2 molecule In order to find the total valence electrons in CS2 (carbon disulfide) molecule, first of all you should know the valence electrons present in carbon atom as well as sulfur atom.

CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is sometimes used to indicate a shared pair of electrons: A single shared pair of electrons is called a single bond. 3.1: Lewis Structures. Chemical bond refers to the forces holding atoms together to form molecules and solids. This force is of an electric nature, and the attraction between electrons of one atom to the nucleus of another atom contributes to what is known as chemical bonds.




