Molar mass is the mass (in atomic mass units) of one mole of a of a substance. One atomic mass unit (u) is equal to 1/12 the mass of one atom of carbon-12. It is also sometimes called: Molecular Mass, Molecular Weight, Formula Mass, or Formula Weight. How can I find the molar mass of an element? The molarity calculator calculates the mass of compound required to achieve a specific molar concentration and volume. To dilute a solution of known molarity, please use the Solution Dilution Calculator. To dilute a solution of concentrated acid or base of known w/w% strength, please use the Acid & Base Molarity Calculator. Formula weight g/mol
Molar Mass Calculator Inch Calculator
Molar mass μ is a physical quantity that tells us what the mass of one mole of a substance is. We can calculate it in a simple way by dividing the mass of the substance m by its amount in moles n: μ = m/n The SI unit of molar mass is kg/mol, but the g/mol unit is more commonly used. Molar mass vs. molecular weight Molecular Weight Calculator This online calculator you can use for computing the average molecular weight (MW) of molecules by entering the chemical formulas (for example C3H4OH (COOH)3 ). This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration (or recalculating grams per ml to moles). You can also calculate the mass of a substance needed to achieve a desired molarity. This article will provide you with the molarity definition and the molarity formula. Molarity Calculator The calculators on this page are independent and can be used in any order. The calculators are numbered because sometimes the results of one calculator are used as inputs to a later one. 1. Mass from volume & concentration Concentration: Formula Weight (daltons): Volume: Mass = 2. Volume from mass & concentration Mass:

How to Find Molecular Mass
This can be done using our molar mass calculator or manually by following our tutorial. In our prior example: Molecule Coefficient Molar Mass; MgCl2: 1: 95.211:. To convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass to get moles. For example, lets say we have 100g of MgCl2 and. More. Embed this widget ». Added Jan 31, 2013 by stuchalk in Chemistry. This is example widget calculates the molar mass of a compound given its chemical formula. Send feedback | Visit Wolfram|Alpha. Molar mass of. Submit. Get the free "Molar Mass Calculator" widget for your website, blog, Wordpress, Blogger, or iGoogle. Molar Mass Calculator. Enter the formula and press "calculate" to work out the molecular mass, the number of moles in 1 g and the percentage by mass of each element. This calculator can be downloaded for off-line use - see below. Chemical symbols are case specific and should be entered correctly to prevent ambiguity - for example, Fe will work. Molar mass ( molar weight) is the mass of one mole of a substance and is expressed in g/mol. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. One mole contains exactly 6.022 ×10 23 particles (Avogadro's number) Steps to calculate molar mass

How to Calculate Molar Mass 6 Steps (with Pictures) wikiHow
Method 1 Calculating the Molar Mass of an Element Download Article 1 Understand molar mass. Molar mass is the mass (in grams) of one mole of a substance. [3] Using the atomic mass of an element and multiplying it by the conversion factor grams per mole (g/mol), you can calculate the molar mass of that element. 2 A Moles to Mass Calculator is a tool used in chemistry to convert the quantity of moles of a substance to its corresponding mass in grams. This conversion is important for chemical reactions, stoichiometry calculations, and understanding the relationship between the amount of a substance and its mass.
Molecular mass is the mass of a single molecule of a compound. It can be calculated by summing the atomic masses of each nuclide present in the molecule and is measured in Daltons (Da or u). The atomic mass of all the known chemical elements can be found in the periodic table. m = n * MM m = n ∗ MM Where m is the Mass From Moles (g) n is the amount of substance (moles) MM is the molar mass (g/mole) To calculate the mass from moles, multiply the number of moles by the molar mass. How to Calculate Mass From Moles? The following example problems outline how to calculate Mass From Moles. Example Problem #1:
Al molar mass htpastor
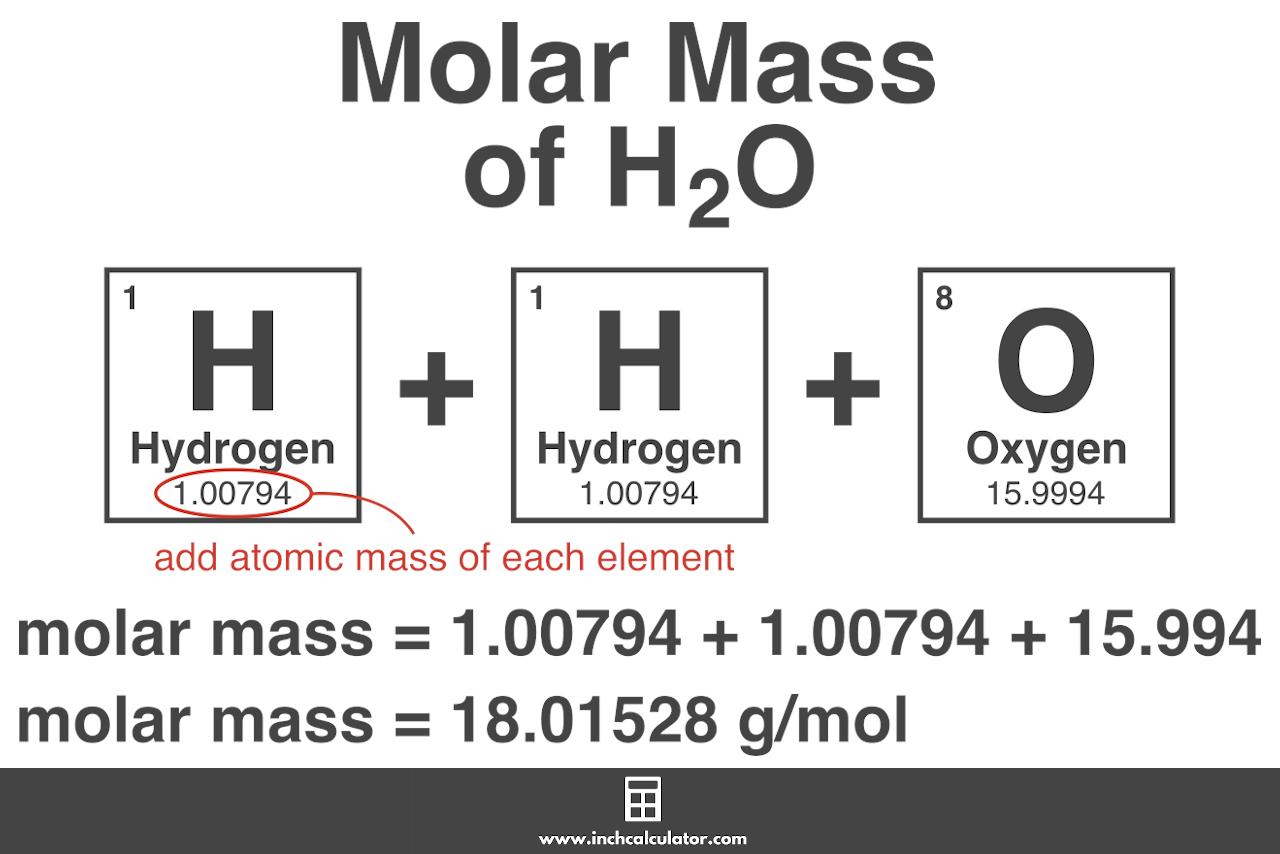
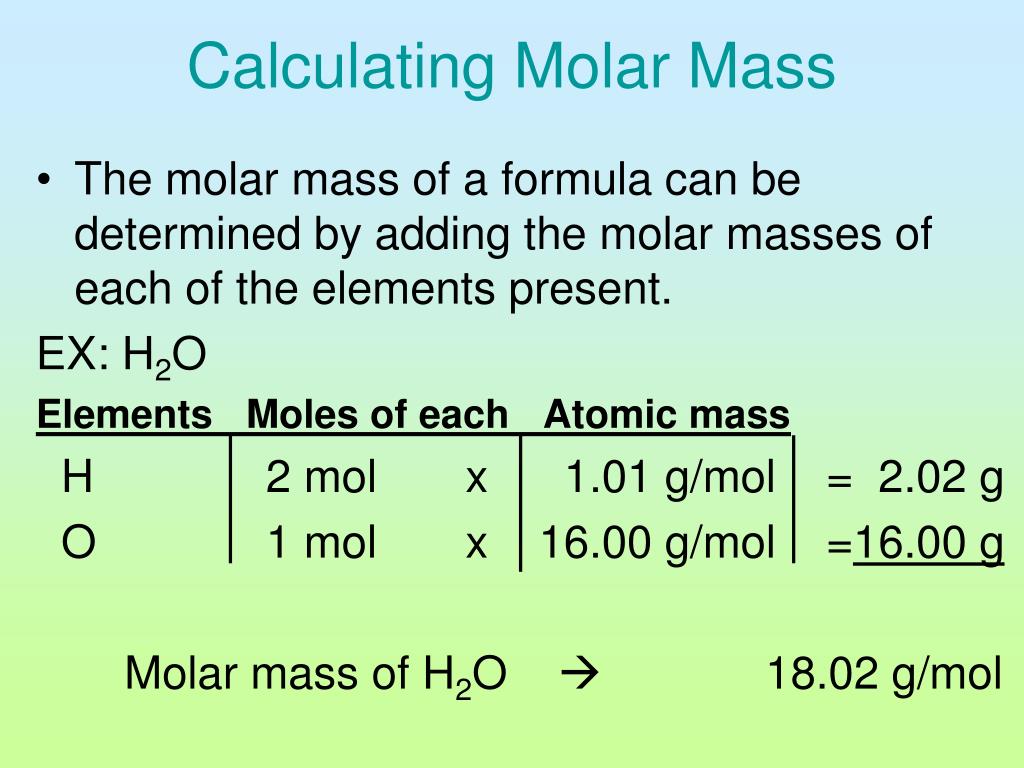
MOLAR MASS CALCULATOR. Enter a chemical formula to calculate its molar mass (e.g. Fe4 [Fe (CN)6]3, NaHCO3, ch3coonh4, h2so4, pb (c2h3o2)2*3h2o, caso4*1/2h2o) and press Enter or click Calculate button. The relative atomic mass indicates how many times larger the mass of a given atom is than 1/12 the mass of the 12 C carbon isotope. The atomic mass of hydrogen is 1.00794 and oxygen is 15.9994. The formula to calculate the molecular weight based on the atomic weights looks like this: molar mass = 1.00794 (H) + 1.00794 (H) + 15.9994 (O) Therefore, the molar mass of H 2 O is 18.01528 g/mol . Learn more about calculating the weight of H 2 O on our water weight calculator .




