A step-by-step explanation of how to draw the Methanol Lewis Dot Structure.In the Lewis structure of structure there are a total of 14 valence electrons. is. Steps to Draw the Lewis Structure of Methanol (CH3OH) 1) Calculation of total valence electrons in methanol The total no. of valence electrons in a molecule is the sum of valence shell electrons of each element in the molecule. Take a look at the following table for the same.
Lewis dot structure for methanol YouTube
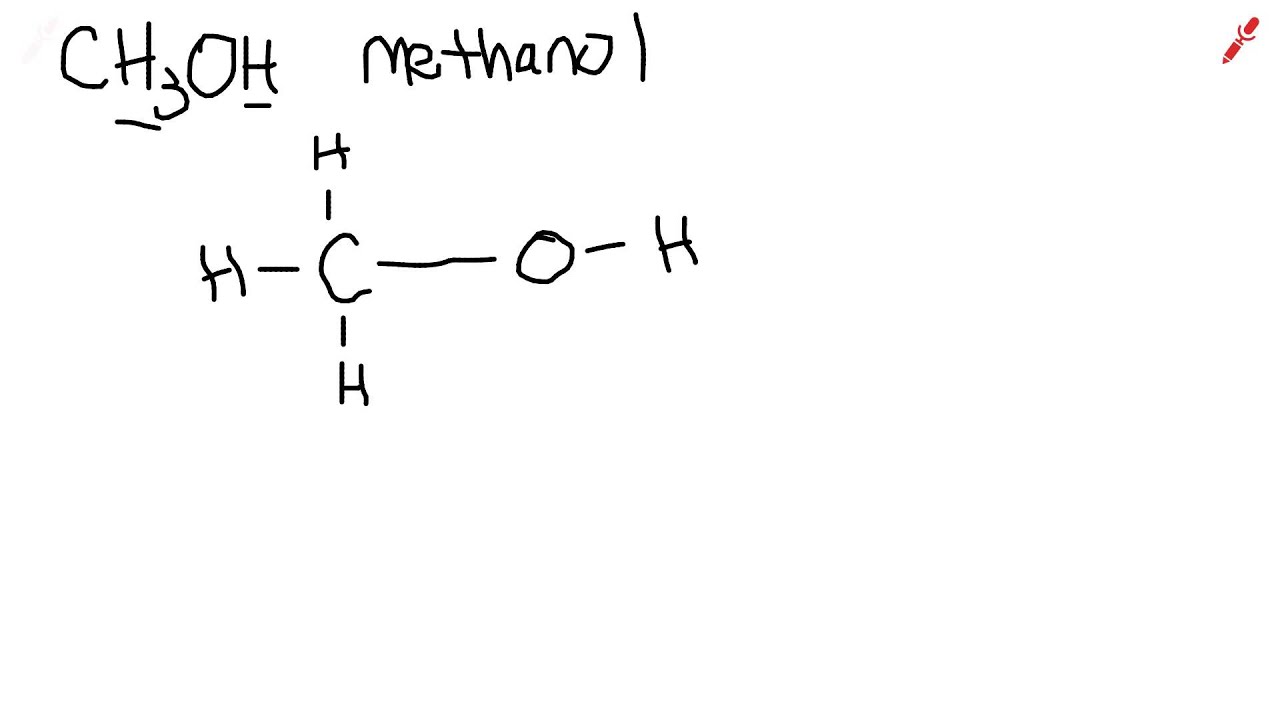
How to Draw the Lewis Structure for CH3OH (Methanol) Wayne Breslyn 725K subscribers Join Subscribe Subscribed 546 Share 136K views 10 years ago A step-by-step explanation of how to draw the CH3OH. Lewis Dot Structure of CH3OH (Methanol) kentchemistry.com 25.1K subscribers Subscribe Subscribed 127K views 12 years ago Every Video I quickly take you through how to draw the Lewis. Methanol (CH 3 OH) is the simplest alcohol which has only one carbon atom. According to the lewis structure of methanol, it has one O-H bond, three C-H bonds and one C-O bond. There are 2 lone pairs on oxygen atom. There are total of 14 electrons in valence shells in the overall molecule as lone pairs and bonds. CH 3 OH lewis structure Consider the Lewis structure of methanol, CH 3 OH (methanol is the so-called 'wood alcohol' that unscrupulous bootleggers sometimes sold during the prohibition days in the 1920's, often causing the people who drank it to go blind). Methanol itself is a neutral molecule,.
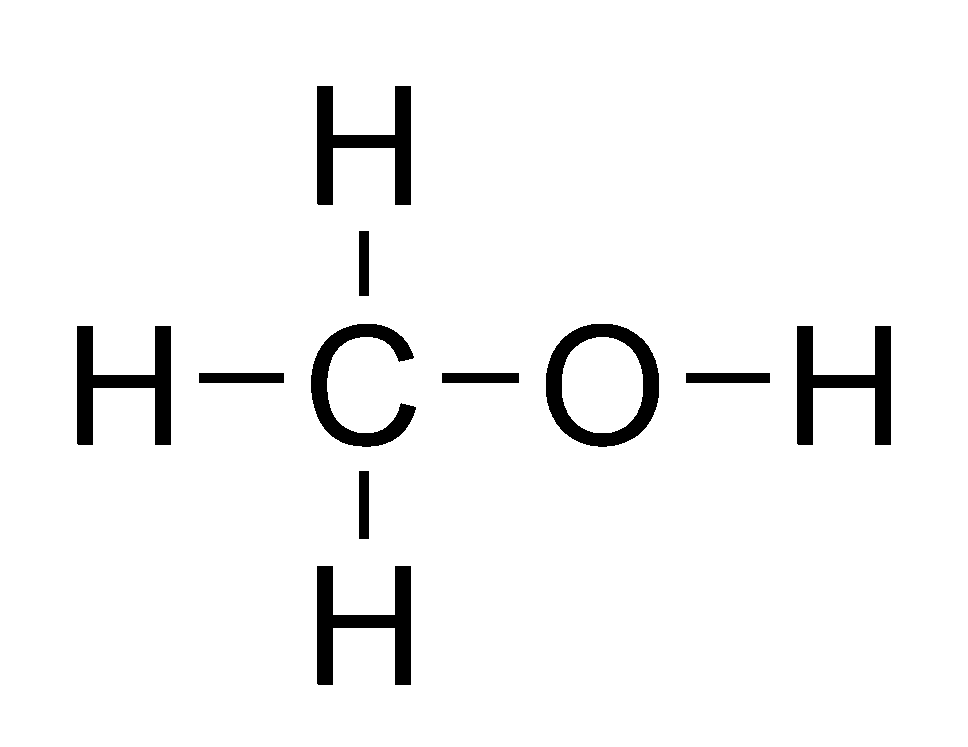
FileMethanol flat structure.png Wikimedia Commons
Consider the Lewis structure of methanol, CH 3 OH (methanol is the so-called 'wood alcohol' that unscrupulous bootleggers sometimes sold during the prohibition days in the 1920's, often causing the people who drank it to go blind). Methanol itself is a neutral molecule,. Consider the Lewis structure of methanol, CH 3 OH (methanol is the so-called 'wood alcohol' that unscrupulous bootleggers sometimes sold during the prohibition days in the 1920's, often causing the people who drank it to go blind). Methanol itself is a neutral molecule,. The Lewis structure of CH3OH, also known as methanol, is a representation of the molecule's bonding and electron distribution. It provides valuable insights into the molecule's geometry, hybridization, and polarity. Let's explore the step-by-step process of determining the Lewis structure of CH3OH. Calculation of Valence Electrons Lewis dot structure is a pictorial representation of the molecule, it's bonding with other atoms and the arrangement of atoms in the compound. It helps in knowing the number of bonded electrons, lone pairs, and the compound's molecular shape.
Methanol Molecule Illustration Stock Illustration Illustration of
The Lewis diagram and the bonded structure are shown in the Methanol Lewis dot structure graphic. Activities. In this quick lab, students will build the methanol molecule and run a basic computational chemistry calculation (using the Hartree-Fock method with 3-21G mathematics, using the Gaussian software) to calculate where the electrons are. 404 71K views 4 years ago A quick explanation of the molecular geometry of CH3OH (Methanol) including a description of the CH3OH bond angles..more.more How to Determine Bond Angle with.
Lewis Dot of Methanol (methyl Alcohol) CH 3 OH Back 70 More Lewis Dot Structures Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). October 31, 2023 by Deep. The information on this page is fact-checked. CH 3 OH Lewis structure. CH 3 OH (methanol) has one carbon atom, four hydrogen atoms, and one oxygen atom. In the CH 3 OH Lewis structure, there are three C — H bonds, one C — O bond, and one O — H bond. And on the oxygen atom, there are two lone pairs.

Methanol (CH3OH) Fisher Scientific
Illustrated Glossary of Organic Chemistry Methan ol ( methyl alcohol ; Me OH ; wood alcohol ): The simplest alcohol , CH 3 OH. Commonly used as a polar protic solvent ( ε = 33). Molecular Structure of Methan ol Step #1: Calculate the total number of valence electrons. Here, the given molecule is CH3OH (methanol). In order to draw the lewis structure of CH3OH, first of all you have to find the total number of valence electrons present in the CH3OH molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).




