SF2 is polar in nature because the sulfur (2.58) and fluorine (3.98) atoms in the molecule differ in their electronegativity and the molecule has a bent geometrical shape. Therefore, the dipoles of the S-F bond do not cancel out each other and molecules turn out to be polar and contribute some dipole moment. Is SF2 Polar or Nonpolar? Wayne Breslyn 665K subscribers Subscribe 33K views 9 years ago If you look at the Lewis structure for SF2 might appear to be a symmetrical molecule. However, according.

Sf2 polar or nonpolar limfamike
Contents SF2 Valence electrons SF2 Lewis Structure SF2 Hybridization SF2 Molecular Geometry SF2 Shape SF2 Bond Angles Is SF2 polar or nonpolar? SF2 Valence electrons For drawing the Lewis structure for any molecule, we first need to know the total number of valence electrons. Page Contents show How to draw lewis structure of SF2? SF2 Lewis structure is made up of two atoms, sulfur (S), and fluorine (F), the sulfur (S) is in the central position and fluorine (F) atoms are on either side of it. The lewis structure of SF2 contains 16 nonbonding electrons and 4 bonding electrons. Hey Guys!In this video, we are going to determine the polarity of Sulphur Difluoride having a chemical formula of SF2. It is made of one Sulphur atom and two. Is the SF2 molecule polar or nonpolar? SF2 is a polar molecule because sulfur has extra electrons that push the molecule into a bent shape because of electron push. 7. What are the bond angles in the SF2 molecule? In the SF2 molecule, the angles between the bonds are approximately 98 degrees. This molecule has a bent or V-shaped molecular.

Is SF2 Polar or Nonpolar? (Sulfur Difluoride) Polar, Chemical formula
SF2 lewis structure is an electron dot representation which can explain many other characteristics related to it. Discover a step by step method to generate SF2 lewis structure is of significance. How to draw the lewis structure for SF2? Count the number of valence electrons (Explained in 3 Steps) SF2 is a polar molecule because it has poles of partial positive charge (ẟ+) and partial negative charge (ẟ-) on it. Let me explain this to you in 3 steps! Step #1: Draw the lewis structure Here is a skeleton of SF2 lewis structure and it contains two S-F bonds. In SF2 lewis structure, the sulfur atom possesses two bonding pairs of electrons and two nonbonding pairs of electrons that reflect the VSEPR idea of AX2E2, which correlates to an angular/non-linear or bent molecular geometry. As a result, Sulfur Difluoride has a bent molecular geometry. The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.
Sf2 polar or nonpolar lenaka
Sulfur difluoride (SF2) is a polar molecule. The central sulfur (S) atom in SF2 is surrounded by two fluorine (F) atoms forming a bent or V-shaped molecule. A fluorine (F) atom is more electronegative than a sulfur (S) atom. Thus both S-F bonds are individually polar in the SF2 molecule and possess a specific dipole moment value. Explain how a molecule that contains polar bonds can be nonpolar. Answer. As long as the polar bonds are compensated (for example. two identical atoms are found directly across the central atom from one another), the molecule can be nonpolar. PROBLEM \(\PageIndex{2}\)
Is it polar or nonpolar? Explain. Chemistry for Changing Times John W. Hill; Terry W. McCreary; Doris K. Kolb 14 Edition Chapter 4, Problem 60 Question Answered step-by-step The molecule SF2 S F 2 is bent. Is it polar or nonpolar? Explain. Instant Solution: Step 1/4 Step 1: First, we need to draw the Lewis structure of SF2 S F 2. Best Answer. Copy. Think of the sulfite ion as a molecule with its geometry and dipole moment AND a net charge. The electron pair geometry is tetrahedral and the molecular geometry is trigonal.
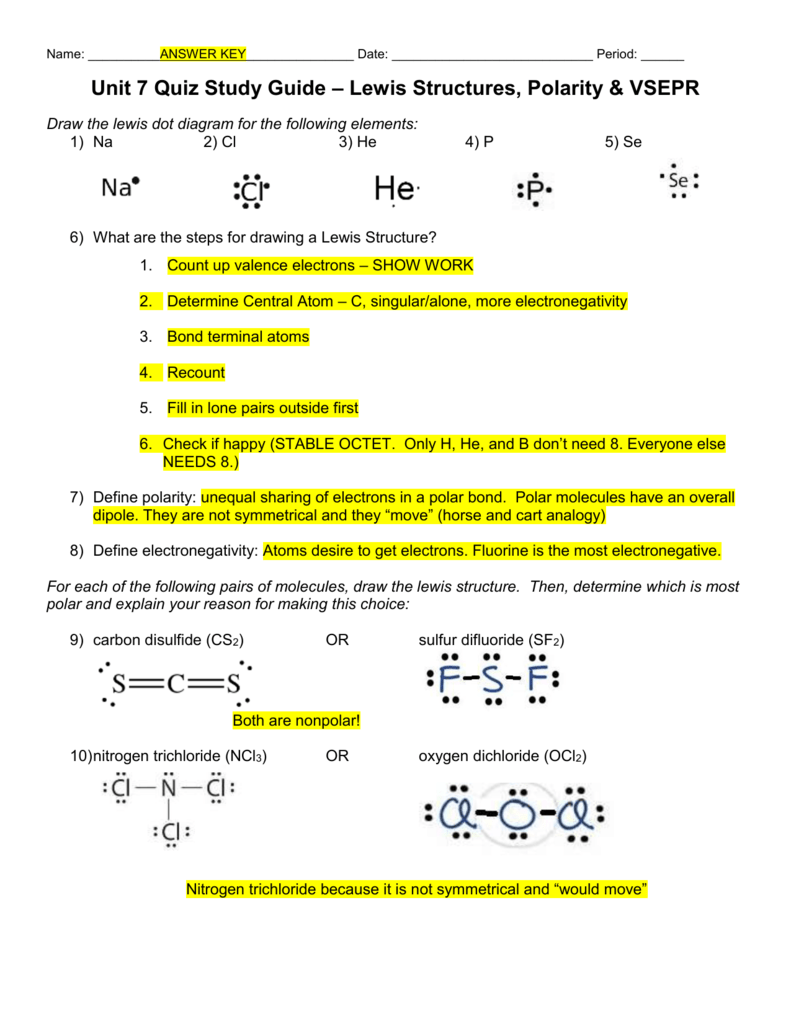
Sf2 polar or nonpolar limfath
0:00 / 1:58 Is SF4 (Sulfur tetrafluoride) Polar or Non-Polar? Wayne Breslyn 727K subscribers Subscribed 17K views 2 years ago Learn to determine if SF4 (Sulfur tetrafluoride) is polar or. Sulfur difluoride. Molecular Formula F. 2. S. Average mass 70.062 Da. Monoisotopic mass 69.968880 Da. ChemSpider ID 123122.




