The Lewis structure for XeO4 is: As per the above diagram, the octet of all the involved elements is satisfied with all four Oxygen atoms forming a double bond with the Xenon atom while Xenon now having more than 8 electrons in the valence shell is allowed to have an expanded octet, therefore, reducing the formal charge and attaining stability. A step-by-step explanation of how to draw the XeO4 Lewis Dot Structure (Xenon tetroxide).For the XeO4 structure use the periodic table to find the total numb.

XeO4 lewis structure, Molecular geometry, Polar or nonpolar, Hybridization
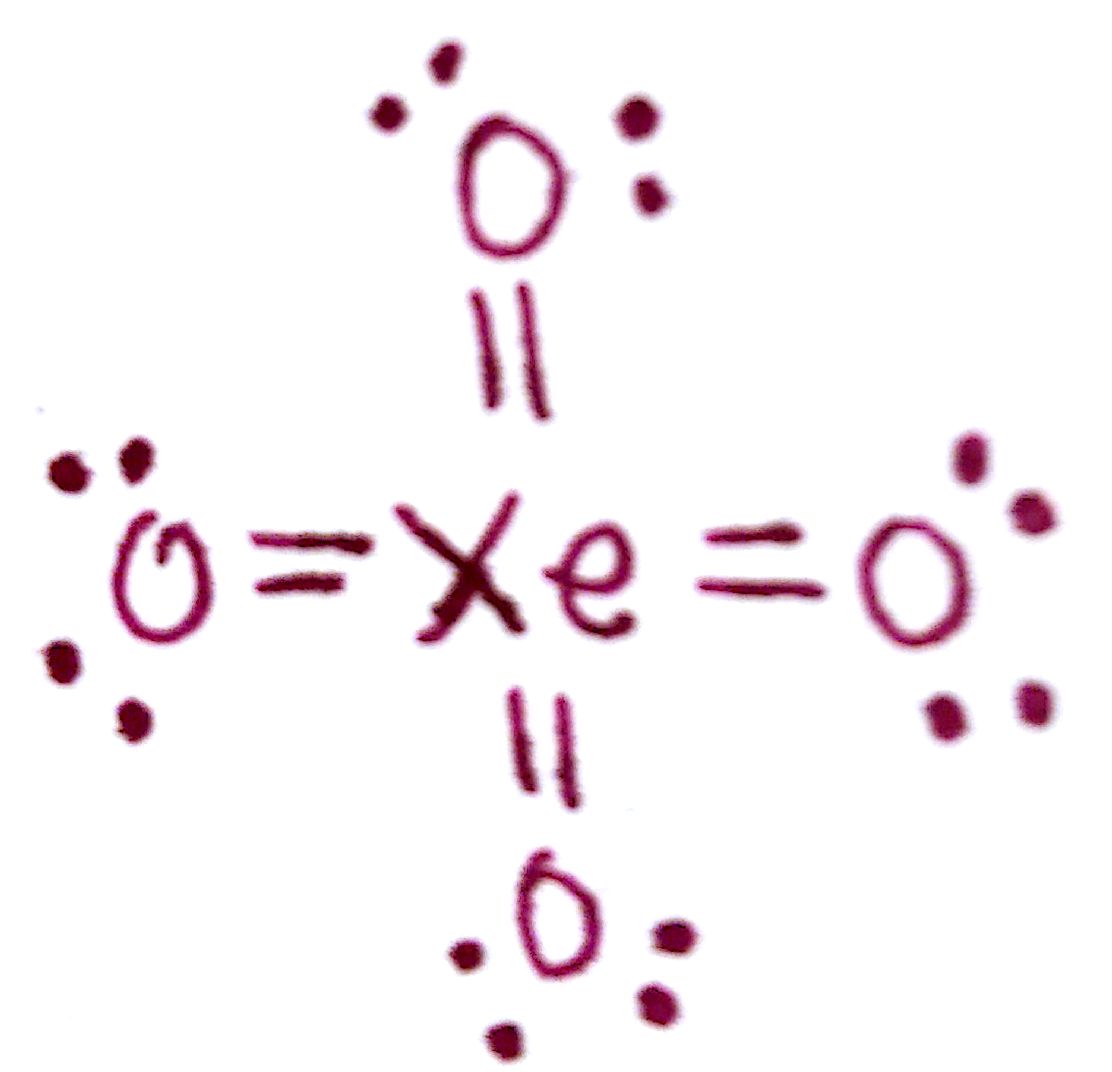
Xenon tetroxide is a chemical compound of xenon and oxygen with molecular formula XeO 4, remarkable for being a relatively stable compound of a noble gas. It is a yellow crystalline solid that is stable below −35.9 ° C; above that temperature it is very prone to exploding and decomposing into elemental xenon and oxygen (O 2 ). [4] [5] The XeO4 lewis structure has tetrahedrally shaped having a bond angle of 109.50. It is a very stable compound of a noble gas which is a very exceptional case. Due to the tetrahedral geometry, the central Xe is sp3 hybridized. An explanation of the molecular geometry for the XeO4 (Xenon tetroxide) including a description of the XeO4 bond angles. The electron geometry for the Xenon. In the XeO 4 Lewis structure, there are four double bonds around the xenon atom, with four oxygen atoms attached to it, and on each oxygen atom, there are two lone pairs. XeO4 Lewis Structure - How to Draw the Lewis Structure for XeO4 Watch on Contents Steps #1 Draw a rough skeleton structure #2 Mention lone pairs on the atoms

molecular geometry of XeO4 Brainly.in
structure diagram of XeO4 Natural Language Math Input Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports, finance, music… Lewis structure of XeO4 contains four double bonds between the Xenon (Xe) atom and Oxygen (O) atoms. The Xenon atom (Xe) is at the center and it is surrounded by 4 Oxygen atoms (O). All the four Oxygen atoms (O) have 2 lone pairs. Let's draw and understand this lewis dot structure step by step. The molecular structure of XeO 4 has been investigated in the gas phase by electron diffraction. The data are completely compatible with the tetrahedral structure proposed from analysis of the infrared spectrum. Geometry XeO4 Geometry and Hybridization Xe is the central atom, so we can draw a preliminary skeletal structure: There are 4×6 + 8 = 32 electrons and this time, instead of putting three lone pairs on the oxygen, we are going to directly add double bonds to leave two lone pairs for each oxygen:
Xeo3 Lewis Dot Structure
XeO4 lewis structure is made up of one xenon and four oxygen atom, the xenon is in a central position, and all oxygen is at the surrounding position. There are four double bonds (Xe=O) present in the XeO4 lewis structure. The lewis structure of XeO4 contains 8 pairs of nonbonding electrons and 8 pairs of bonding electrons. XeO4 lewis structure has a Xenon atom (Xe) at the center which is surrounded by four Oxygen atoms (O). There is a double bond between the Xenon (Xe) & all the four Oxygen (O) atoms. There are 2 lone pairs on the Oxygen atoms (O).
In resonance structure A, the xenon and oxygens are neutral; while in resonance structure B, the xenon has a charge of +4 and each oxygen has a charge of -1. The $\ce{Xe-O}$ bonds in $\ce{XeO4}$ are very weak. In resonance structure A the pi overlap is very poor due to the different atomic sizes of xenon and oxygen. The molecular structure of XeO4 has been investigated in the gas phase by electron diffraction. The data are completely compatible with the tetrahedral structure proposed from analysis of the infrared spectrum. Refinement of the structure by least squares based upon intensity functions, treating each distance and amplitude as independent parameters, yielded the results rXe-O = 1.736 A (0..

Incredible Xeo4 Lewis Structure Ideas
Xenon tetroxide (XeO 4) is an unusual noble gas compound. It contains xenon in its highest possible oxidation state (+8). Its yellow crystals are stable below -36 °C, but it decomposes explosively above that temperature. It must be handled under strict safety precautions. XeO 4 's tetrahedral geometry follows valence shell electron pair. A step-by-step explanation of how to draw the XeOF4 Lewis Dot Structure (Xenon oxytetrafluoride).For the XeOF4 structure use the periodic table to find the t.




