Riesenauswahl an Markenqualität. Folge Deiner Leidenschaft bei eBay! Kostenloser Versand verfügbar. Kauf auf eBay. eBay-Garantie! 5.10: Bohr Model of the Atom; 5.11: Energy Levels and Sublevels; 5.E: Models of the Atom (Exercises) 5: Models of the Atom is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Back to top; 4.11: Potential and Kinetic Energy; 5.1: Electron Configuration;

Atom tlenu — Zdjęcie stockowe © bobyramone 7428861
Tlen ( O, łac. oxygenium) - pierwiastek chemiczny o liczbie atomowej 8, niemetal z grupy tlenowców w układzie okresowym . Stabilnymi izotopami tlenu są 16 O (stanowi ponad 99% tlenu naturalnego), 17 O oraz 18 O . Tlen w stanie wolnym występuje w postaci cząsteczek dwuatomowych O 2 oraz trójatomowych - ozonu O 3 (głównie w ozonosferze ). Rutherford model, description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called electrons, circulate at some. The crystal diffusion variational autoencoder (CDVAE) is a machine learning model that leverages score matching to generate realistic crystal structures that preserve crystal symmetry. In this. The current theoretical model of the atom involves a dense nucleus surrounded by a probabilistic "cloud" of electrons. Atomic theory is the scientific theory that matter is composed of particles called atoms.The concept that matter is composed of discrete particles is an ancient idea, but gained scientific credence in the 18th and 19th centuries when scientists found it could explain the.
Atom tlenu Model 3D TurboSquid 1195563
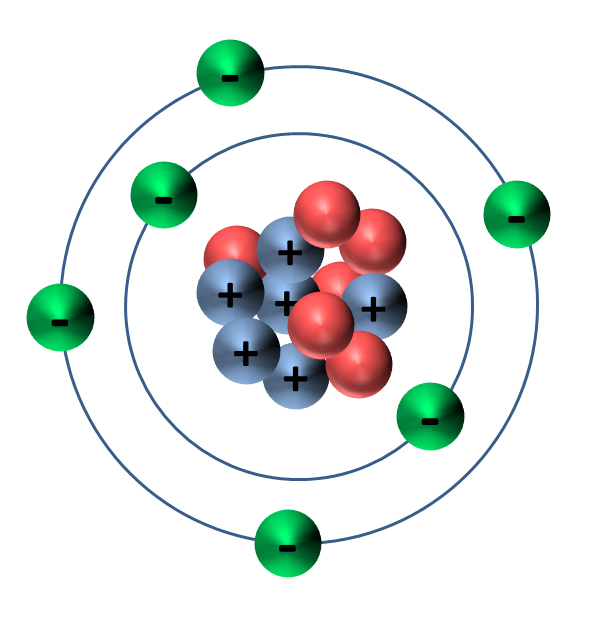
Figure 4.1.3 4.1. 3: The Bohr model of the atom illustrating levels of electrons. This planetary model of the atom was attractive to scientists because it was similar to something with which they were already familiar, namely the solar system. Unfortunately, there was a serious flaw in the planetary model. Key Takeaways: Model of the Atom. An atom is a building block of matter that cannot be broken apart using any chemical means. Nuclear reactions can alter atoms. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Protons and neutrons form the atomic nucleus. The familiar periodic table of elements with each kind of atom shown as colored balls. The size of each atom in the CPK model (above) is larger than those in the Z-correlated model (below), as it. Build an Atom - PhET Interactive Simulations
Climate Science Investigations South Florida Causes of Climate Change
Thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by William Thomson (Lord Kelvin) and strongly supported by Sir Joseph John Thomson, who had discovered (1897) the electron, a negatively charged part of every atom.Though several alternative models were advanced in the 1900s by Kelvin and others, Thomson held that atoms are uniform. Atomically precise Au and Ag nanoclusters doped with single atom as model alloy catalysts S. Masuda, K. Sakamoto and T. Tsukuda, Nanoscale, 2024, Accepted Manuscript , DOI: 10.1039/D3NR05857C This article is licensed under a Creative Commons Attribution 3.0 Unported Licence. You can use material from this article in other publications without requesting further permissions from the RSC.
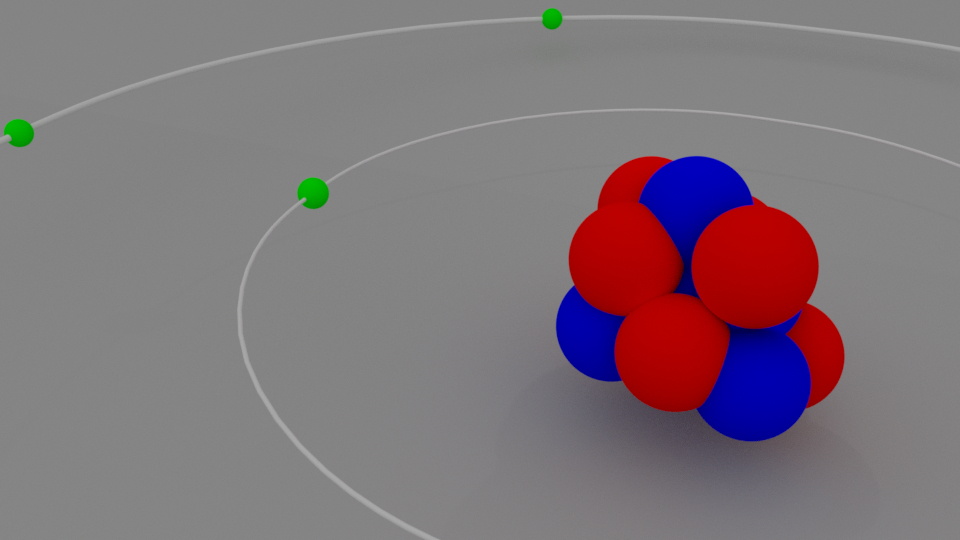
The modern model of the atom. Today, scientists use an atomic model that has a central, positively-charged nucleus that contains: positively-charged protons close proton Subatomic particle with a. 24/7 czat na żywo. Model 3D nauka chemia atom. This is an oxygen atom. Formed by 8 protons, 8 electrons and 8 neutrons. The electronic configuration of this element is 1s2, 2s2, 2p4. nauka medyczny Elektron komórka orbita Elementy protony neutrony tlen. Zgłoś treść.
Atom tlenu Model 3D TurboSquid 1195563
The planetary model of the atom pictures low-mass electrons orbiting a large-mass nucleus. The sizes of the electron orbits are large compared with the size of the nucleus, and most of the atom is a vacuum. The model is analogous to how low-mass planets in our solar system orbit the large-mass Sun. In the atom, the attractive Coulomb force is. This graphic takes a look at the key models proposed for the atom, and how they changed over time. Though our graphic starts in the 1800s, the idea of atoms was around long before. In fact, we have to go all the way back to Ancient Greece to find its genesis. The word 'atom' actually comes from Ancient Greek and roughly translates as.




