When setting up the conversion, worry about one unit at a time, for example, convert the pound units to gram units, first: Next, convert in 2 to cm 2. Set up the conversion without the exponent first, using the conversion factor, 1 in = 2.54 cm. Since we need in 2 and cm 2, raise everything to the second power: Updated on October 04, 2019 Unit conversions are important in all sciences, although they may seem more critical in chemistry because many calculations use different units of measurement. Every measurement you take should be reported with the proper units.
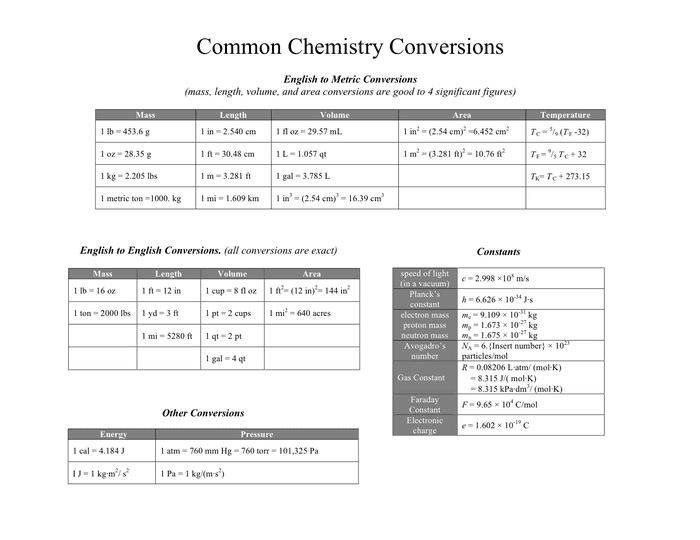
Common chemistry conversions in Word and Pdf formats
Basic Conversion Cheat Sheet • Three basic units of measurement length, mass (weight), volume o The basic unit of length is: METER o The basic unit of volume is: LITER o The basic unit of mass (weight) is: GRAM • The following are some of the prefixes for the metric system. They are based on Here is what we would have gotten: 3.55 m × 1 m 100 cm = 0.0355m2 cm 3.55 m × 1 m 100 c m = 0.0355 m 2 c m. For the answer to be meaningful, we have to construct the conversion factor in a form that causes the original unit to cancel out. Figure 2.6.1 2.6. 1 shows a concept map for constructing a proper conversion. 1, 000 ms 1 s or 1 s 1, 000 ms. To convert 18 ms to seconds, we choose the conversion factor that will cancel out milliseconds and introduce seconds. The conversion factor on the right is the appropriate one. We set up the conversion as follows: 18ms × 1 s 1, 000 ms = 0.018 s. Footnotes 1 Strictly speaking, the ounce and pound are units of weight, W (a force equal to the product of mass and gravitational acceleration, W = mg). The conversion relations in this table are commonly used to equate masses and weight assuming a nominal value for g at the surface of the earth.

Metric System Chemistry Unit Conversion Chart leafonsand
Chemistry Conversion Chart. Moles and molecules are not the only conversions that need to be done in chemistry. Here is a simple table with SOME conversion factors. Measurement Unit converters (aka unit calculators) are tools that allow to convert between different units of measurement for the same quantity. This is the most complete collection of unit converters with 39 different physical quantities supported. Click on a quantity name above to access the unit converter. Common Chemistry Conversions English to Metric Conversions (mass, length, volume, and area conversions are good to 4 significant figures) Mass Length Volume Area Temperature 1 lb = 453.6 g 1 in = 2.540 cm 1 fl oz = 29.57 mL 1 in2 = (2.54 cm)2 =6.452 cm2 T°C = 5 9 (T°F - 32) If unit conversion has never made sense to you then please watch this video. I'll explain the concept of unit conversion and do multiple examples explaining.
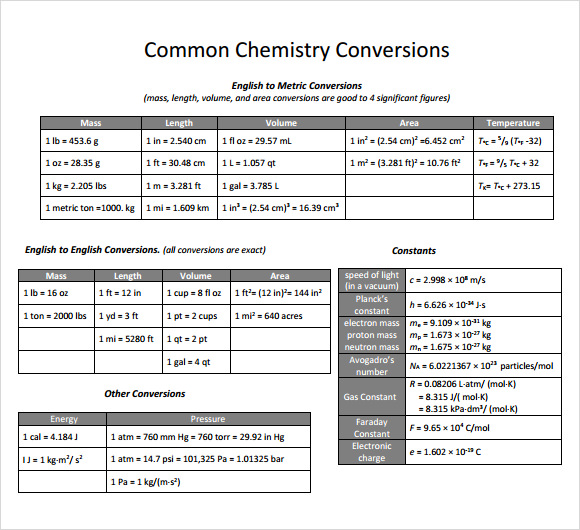
Printable Metric Conversion Chart For Chemistry
Step 2: Calculate. 4.3 cm × 1 m 100 cm × 10 6 μ m 1 m = 43, 000 μ m. Each conversion factor is written so that the unit of the denominator cancels with the unit of the numerator of the previous factor. This calculator converts between the different units used in Chemistry and the Physical Sciences. To use, first choose the unit type from the list below energy energy (per mole and per molecule) and spectroscopic units pressure length angles magnetic moment electric dipole charge mass SI prefixes ©2024 Dr Toby Hudson Please acknowledge its source
Several of the most commonly needed English-metric conversion factors are listed in Table 8.1 on the next page. Because the English inch is defined as 2.54 cm, the number 2.54 in this value is exact. The numbers in the other conversion factors in Table 8.1 are not exact. 2. Table A.2 in Appendix A shows some useful English-English conversion. Perform the following conversions. Note that you will have to convert units in both the numerator and the denominator. 88 ft/s to miles/hour (Hint: use 5,280 ft = 1 mi.) 0.00667 km/h to metres/second; Perform the following conversions. Note that you will have to convert units in both the numerator and the denominator. 3.88 × 10 2 mm/s to.

Si Unit Conversion Chart
From volume (liters) to moles: Divide your initial volume by the molar volume constant, 22.4 L. From moles to volume (liters): Multiply your mole value by the molar volume constant, 22.4L. From particles (atoms, molecules, or formula units) to moles: Divide your particle value by Avogadro's number, 6.02 × 10 23. Learn some helpful tricks on how to remember the metric system, and practice what you just learned to ace your exam! This video helps you understand how to u.




