Answer the following questions. 1. How many atoms are in 6.28 moles of aluminum? 2. How many atoms are in 90.43 moles of copper? 3. How many atoms in 7.64 moles of barium? 4. How many molecules in 3.72 moles of sulfur dioxide? 5. 78.54 g of nitrogen dioxide contain how many molecules? 6. Use a separate piece of paper, show all work, and circle your final answer. (Attach this sheet to your work). Set A: One Step Problems: Convert to moles: 12.04 x 1023 atoms He 3.01 x 1023 atoms Cu 3.612 x 1023 atoms Fe 100 atoms Ar 1 atom S 24 grams C 59.3 grams Sn 98.9 grams Na 5000 grams K 0.005 grams Ne Convert to mass in grams:

14 Mole Conversion Worksheet /
1. N2 + 2O2 → N2O4 a. If 15.0g of N2O4 was produced, how many moles of O2 were required? = 0.326 mol O2 b. If 4.0x10-3 moles of oxygen reacted, how many grams of N2 were needed? = 5.6x10-2 g N2 2. K3PO4 + Al(NO3)3 → 3KNO3 + AlPO4 a. What is the mass of potassium nitrate that is produced when 2.04 moles of potassium phosphate react? = 619g KNO3 b. a. 2.48 g of HBr. b. 4.77 g of CS 2. c. 1.89 g of NaOH. d. 1.46 g of SrC 2 O 4. 16. Decide whether each statement is true or false and explain your reasoning. There are more molecules in 0.5 mol of Cl 2 than in 0.5 mol of H 2. One mole of H 2 has 6.022 × 10 23 hydrogen atoms. The molecular mass of H 2 O is 18.0 amu. answer The only new concept we will introduce in this unit is the idea of a mole. A mole is a quantity of matter that we use for conversion purposes. We can convert from grams to moles, liters to moles (for gases), and atoms or molecules to moles. Title: Relative Mass and the Mole answer key Created Date: 20171005134609Z
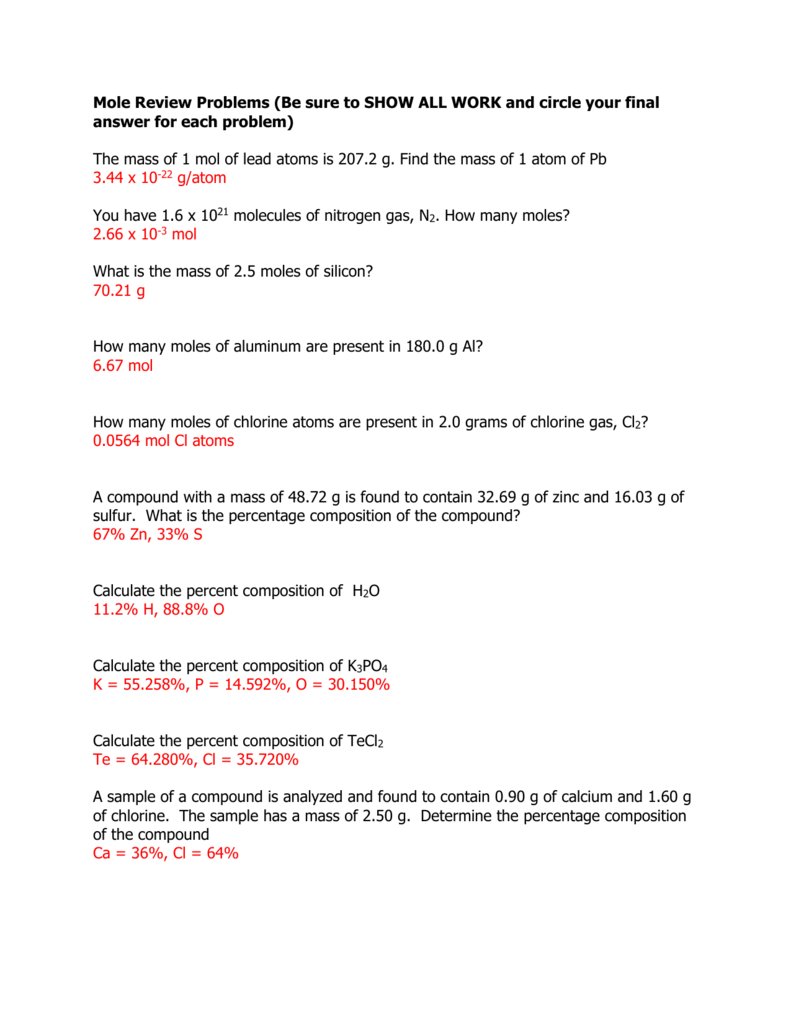
Worksheet Mole Problems Answers
Mole to Mole Problems KEY Worksheet: Mole/Mole Problems Name Answer each of the following questions using the equation provided. BE SURE TO BALANCE EACH EQUATION BEFORE SOLVING ANY PROBLEMS. SHOW ALL WORK. NO + 02 N02 1. How many moles of N - will be produced if 3.6moles NO react? 2. For CO 2 (m.w. = 44.01 u), mole CO 2 = 44.01 g CO 2 = 6.022 x 10 23 CO 2 molecules. Because each CO 2 molecule is composed of one carbon atom and two oxygen atoms, we could say that a mole of CO 2 contains one mole of carbon atoms and two moles of oxygen atoms. In general, it is useful to think of a mole as just an Avogadro's number of things. Moles Molecules and Grams Worksheet How many molecules are there in 24 grams of FeF3? How many molecules are there in 450 grams of Na2S04? 24 How many grams are there in 2.3 x 10 atoms of silver? 23 How many grams are there in 7.4 x 10 molecules of AgN03? 23 How many grams are there in 7.5 x 10 molecules of H2S04? 1 Mass of substance Convert using the molar mass of the substance. 2 Amount of substance in moles Use Avogadro's number for conversion. 3 Number of atoms, molecules, or formula units of substance PROBLEMS INVOLVING ATOMS AND ELEMENTS Sample Problem 1 A chemist has a jar containing 388.2 g of iron filings.

Mole Worksheet 1 Answer Key Printable Word Searches
Answer 0.00119 mol C 4 H 8 O 2 which is 0.105 g C 4 H 8 O 2 7.1.2: Practice Mole Calculations is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Given the following equation, complete the questions below. 8Al + 3Fe3O4 → 4Al2O3 + 9Fe 8 A l + 3 F e 3 O 4 → 4 A l 2 O 3 + 9 F e. determine the number of moles of Fe F e produced from 2.0 moles of Al A l. determine the number of mol of Fe F e produced from 1.0 moles of Fe3O4 F e 3 O 4. determine the number of moles of Al2O3 A l 2 O 3.
The answer keys include very detailed answer keys with all problems set up and solved. This package includes the following 3 resources: Mole Map. Full page COLOR and Black & White versions. 2 maps per page COLOR and Black & White Versions. Mole Conversion Worksheet #1. 10 Conversion Problems (will print 2 per page front & back) The production of ammonia (NH 3) from nitrogen and hydrogen gases is an important industrial reaction called the Haber process, after German chemist Fritz Haber. N 2(g) + 3H 2(g) → 2NH 3(g) The balanced equation can be analyzed in several ways, as shown in the figure below. Figure 12.2.3: This representation of the production of ammonia from.

Mole Calculation Worksheet Answers Mole Calculation Worksheet
NewPathWorksheets offers chemistry study guides, worksheets & answer keys for high schoolers to understand the topic of the Mole. Convenient & printable Mole guides. Answer each of the following questions using the equation provided. BE SURE TO BALANCE EACH EQUATION BEFORE SOLVING ANY PROBLEMS. SHOW ALL WORK. 1. ___NO 2 + ___O2 ___NO2 2 a. 2 moles of NO will react with ______ 1 mole(s) of O2 to produce ______ 2 mole(s) of NO2. b. ? moles NO = 3.6 moles O 2 moles NO 2 2 2 × 1 moles O 2 = 7.2 moles NO2 c.




